Tungsten Trioxide in Lithium-ion Battery

Lithium-ion batteries have become the main power source choice for portable electronic devices due to their large capacity, high voltage, and long life. In order to further satisfy people's demand for higher-performance lithium-ion batteries, it is an effective solution to find alternative electrode materials with better electrochemical characteristics. Tungsten trioxide is a potential lithium ion anode material with a theoretical capacity of 690 mAh g-1. Nano-scale electrode materials have a larger specific surface area and smaller crystal size, which can provide more reactive potential and shorter lithium ion diffusion length, which is beneficial to improve electrochemical performance.
A. Morphology of Tungsten Trioxide
The figure below shows the morphology of tungsten trioxide nanoflowers. As shown in the figure, the full picture of the sample can be seen at a lower magnification. The synthesized product presents a flower-like morphology, and the size of the nanoflower is relatively uniform, and the diameter of the nanoflower is about 5 microns. From the partial scanning electron micrograph of the sample under high magnification, it can be seen that the length of the nanowire constituting the nanoflower is about several hundred nanometers.

The figure below shows the morphology of the nanowire. It can be observed from the low-magnification scanning electron microscope pictures that the length of the nanowire is as long as ten micrometers, and these nanowire are arranged together in a certain direction. Observed from the high magnification scanning electron microscope image, the diameter of the nanowire is relatively uniform, forming a ribbon.

B. Tungsten Trioxide Li-ion Battery Performance
1.Preparation of Lithium-ion Battery Electrode Sheet
In order to study the electrochemical properties of tungsten trioxide nanomaterials, the tungsten trioxide nanomaterials must first be packaged into lithium-ion batteries, and then the electrochemical performance test, the specific steps are as follows: For powdered active materials, usually need to be combined with acetylene black (conductive agent) and PTFE (binder) are configured according to a certain mass ratio (7:2:1). First, pour the weighed active material and acetylene black into a mortar, drop an appropriate amount of NMP (N-methylpyrrolidone) and grind. After the mixture is fully mixed, add PTFE and continue grinding until the three materials are fully mixed to form a uniformly dispersed slurry with a certain viscosity. Then the slurry is evenly coated on the surface of copper foil (negative electrode material) or aluminum foil (positive electrode material), and placed in an oven to fully dry. Finally, use a punching machine to cut the copper foil into round pieces with a diameter of 8 mm for use.。
2.Packaging of Lithium-ion Button Batteries
After completing the preparation of the electrode sheet, the battery can be packaged. The electrode sheet coated with tungsten trioxide is used as the positive electrode (working electrode), the metal lithium sheet is used as the negative electrode (counter electrode and reference electrode), Celgard 2400 microporous polypropylene film is used as the separator, and the electrolyte uses 1M LiPF6 as the solute, and the volume ratio is 1: 1 ethylene carbonate (EC) and dimethyl carbonate (DMC) are solvents. The battery case adopts a stainless steel button battery case model CR2032, and a stainless steel gasket and shrapnel are used as supports inside. During packaging, a lithium ion battery is assembled in the order of positive electrode shell, tungsten trioxide electrode sheet, separator, metal lithium sheet, gasket, shrapnel, and negative electrode shell from bottom to top. Add an appropriate amount of electrolyte into the battery case, and finally cover the battery case, using a sealing machine to seal the battery. All operations are completed in a glove box filled with argon, and the oxygen and water content in the box is strictly limited to less than 1 ppm. The encapsulated battery needs to be allowed to stand and age for 12-24 hours before testing the battery performance.
三、Electrochemical Performance Test
1.Charge and Discharge Curve
The figure below shows the charge and discharge curves of the two electrodes of tungsten trioxide nanoflower and nanowire in the voltage range of 0.01-3.00 V and a current density of 200 mA/g. It can be observed from the figure that the first discharge capacity and charge capacity of nanoflower are 711 mAh g-1 and 196 mAh g-1, respectively. The first discharge capacity and charge capacity of the nanowire are 649 mAh g-1 and 95 mAh g-1, respectively. It can be calculated that the first irreversible capacity loss of nanoflowers and nanowire are 72% and 85%, respectively. The reason for this result is the irreversible process in the first electrochemical reaction of the tungsten trioxide sample and the additional consumption of lithium ions and the decomposition of the electrolyte during the formation of the solid electrolyte interface film. At the beginning of the second cycle, there is almost no loss of nanoflower and nanowire capacity. From the curve in the figure, it can be seen that the charge and discharge capacity of nanoflowers is about 50 mAh g-1 higher than nanowires. Starting from 110 cycles, the cycle performance of nanoflowers is more stable than nanowire. The reversible capacity of tungsten trioxide nanoflowers remained at 135 mAh g-1 after 180 cycles, and the reversible capacity of tungsten trioxide nanowire remained at 97 mAh g-1 under the same test conditions. The reason for this phenomenon may be that the specific surface area of the tungsten trioxide nanoflower is larger than that of the nanowire.
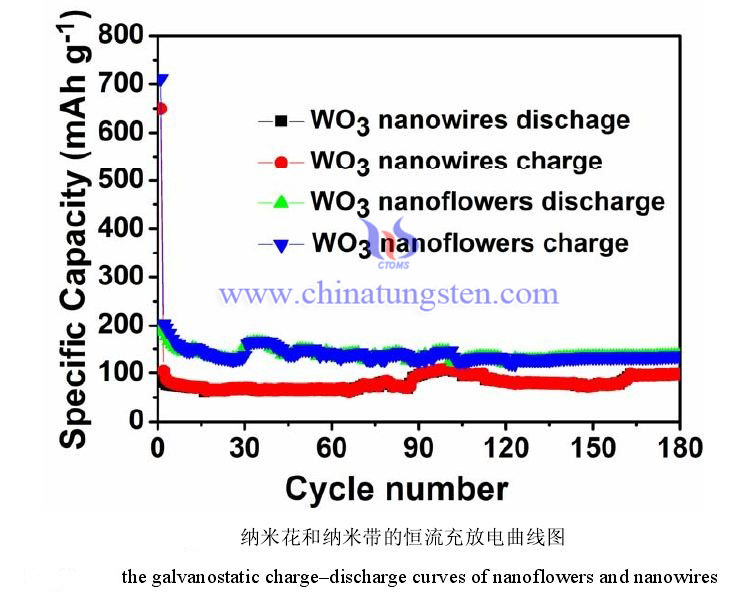
2.Rate Performance
The rate performance reflects the performance of the battery during charging and discharging at different current densities. Generally speaking, the higher the current, the faster the charging and discharging, and the more insufficient the charging and discharging of the battery. The actual measured battery capacity will decrease compared with the low current. Therefore, the rate performance is an important indicator to measure the excellent performance of the battery in a high-power working state. In the actual test, you only need to modify the current value during the constant current charge and discharge test to obtain a series of data. The figure below is the rate performance test chart of nanoflowers and nanowire at different current densities. The test current density is 200 mAh g-1, 400 mAh g-1, 800 mAh g-1 and 1600 mAh g-1, and then the current density is reduced back to 200 mAh g-1. The number of cycles tested under each current is 10 cycles. This test method not only reflects the capacity of the electrode material under different current densities, but also shows whether it can return to the initial low current capacity after high current charge and discharge. It can be seen from the figure that the capacity of the nanoflower is smaller than the initial capacity when it returns to the current density of 200 mAh g-1. This may be due to changes in the volume of the nanoflowers during charging and discharging at high current densities. When the current returned to 200 mAh g-1 again, the capacity of the nanoribbons sample hardly changed, and there was a good capacity retention. It can also be observed from the figure that both have excellent rate stability performance.
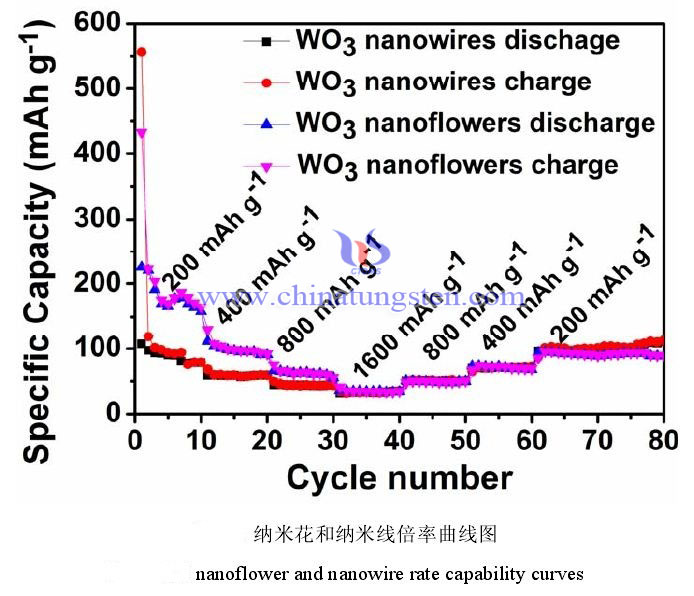
It can be concluded that the two structures have good cycling ability and stability under constant current. The reversible capacity of tungsten trioxide nanoflowers remained at 135 mAh g-1 after 180 cycles, and the reversible capacity of tungsten trioxide nanowire remained at 97 mAh g-1 under the same test conditions. The capacity of nanoflowers is higher than that of nanowire, which may be attributed to the larger specific surface area of tungsten trioxide nanoflowers than nanowire. The rate performance tests of the two structures show that both nanowire and nanoflower have good rate stability.




